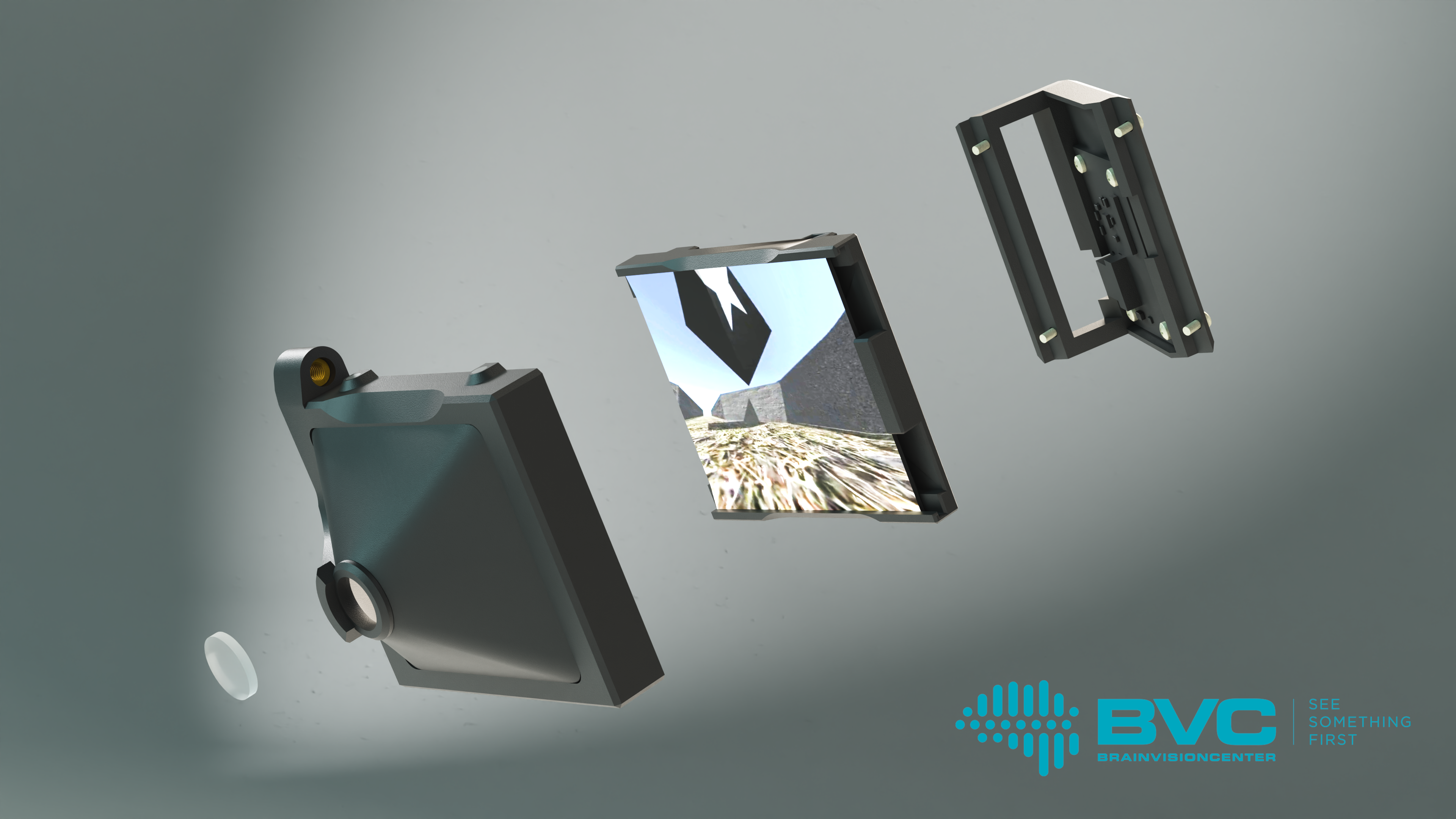
The BrainVisionCenter Research Institute and Competence Center (BVC), in collaboration with the HUN-REN Institute of Experimental Medicine (HUN-REN KOKI), has developed a virtual reality (VR) headset optimized for mice. This groundbreaking device creates new avenues for studying brain function and advancing brain-computer interfaces aimed at restoring vision. The device, named “Moculus,” provides experimental animals with a highly realistic simulation of natural vision, enabling up to 100 times faster learning. The innovation aligns with the mission of the BVC, founded by Botond Roska and Balázs Rózsa, which focuses on developing vision-restoring therapies and treatments for central nervous system diseases. The development is the work of Linda Judák, Gergely Szalay, Gergely Dobos, and Balázs Rózsa, who examined the plasticity of the mouse visual cortex during rapid learning. The significance of their research is underscored by its publication in one of the world’s most prestigious scientific journals, Nature Methods.
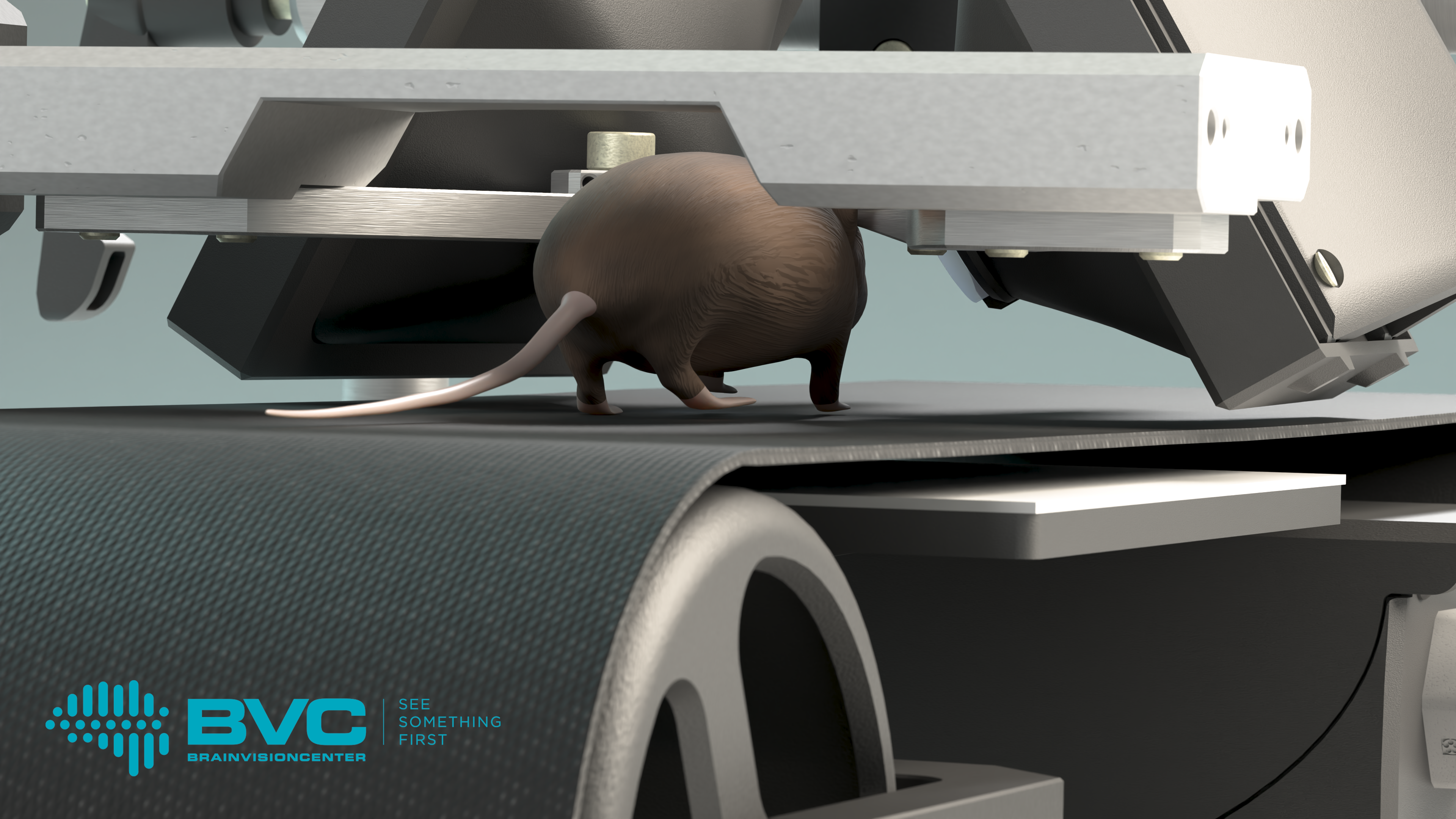
The cortical representation of behavior and perception is one of the key areas of scientific research, as it forms the foundation for a deeper understanding of brain mechanisms and the discovery and development of related therapeutic strategies. To understand these phenomena at the level of individual cells, the most appropriate method is to study the brain activity of mice using real-time 3D imaging. However, during such studies, it is particularly important to keep the mouse’s head completely stable, as movement can compromise the accuracy of the results. Researchers typically achieve this by fixing the mouse’s head and employing virtual reality systems.
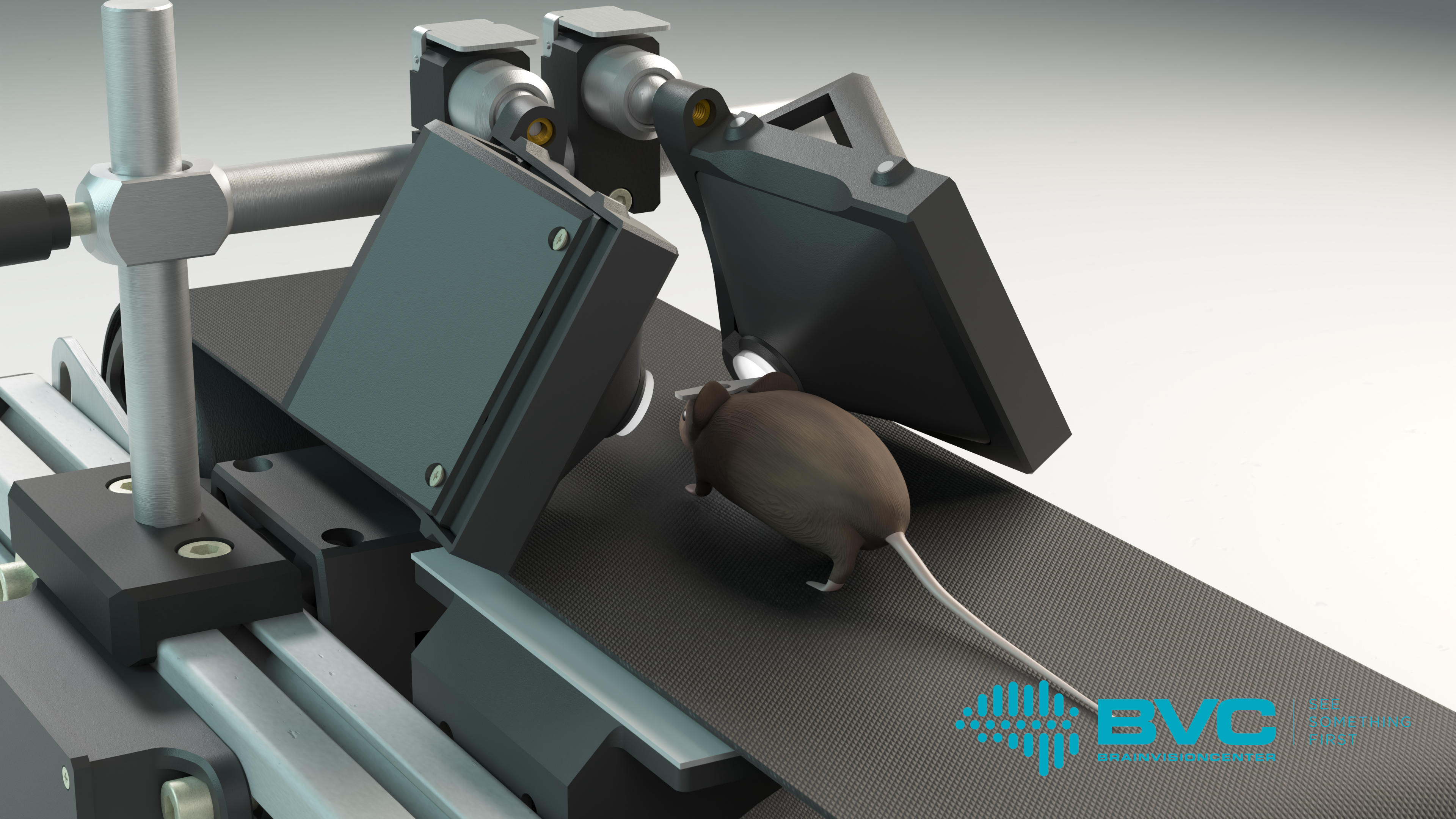
Over the past 20–30 years, neuroscientists, pharmaceutical companies, and corporations have developed numerous virtual reality tools to study the vision of experimental animals. However, these tools typically used two-dimensional projections to represent virtual spaces, assuming that experimental animals, like humans, are capable of reconstructing the surrounding 3D reality from two-dimensional images, such as humans do for the flat image of a television screen. Researchers from HUN-REN KOKI, BVC, the Institute of Molecular and Clinical Ophthalmology Basel, and Pázmány Péter University, however, have demonstrated that this assumption is incorrect. For rodents, two-dimensional projections do not provide a realistic experience, which can distort behavior results. This was illustrated by Gergely Dobos and his colleagues with a simple yet striking example: mice crossed a virtual abyss displayed on traditional VR screens without hesitation. In contrast, they immediately stopped and even retreated backward when the abyss was presented using the Moculus system.
“The project has proven that mice can only perceive the world in three dimensions if virtual reality is projected in a realistic way tailored to their vision. Unlike humans, mice lack sufficient capacity for abstract visual thinking, making it essential for what they see to faithfully reflect reality,” explained dr. Gergely Szalay, lead researcher at HUN-REN KOKI and BVC.
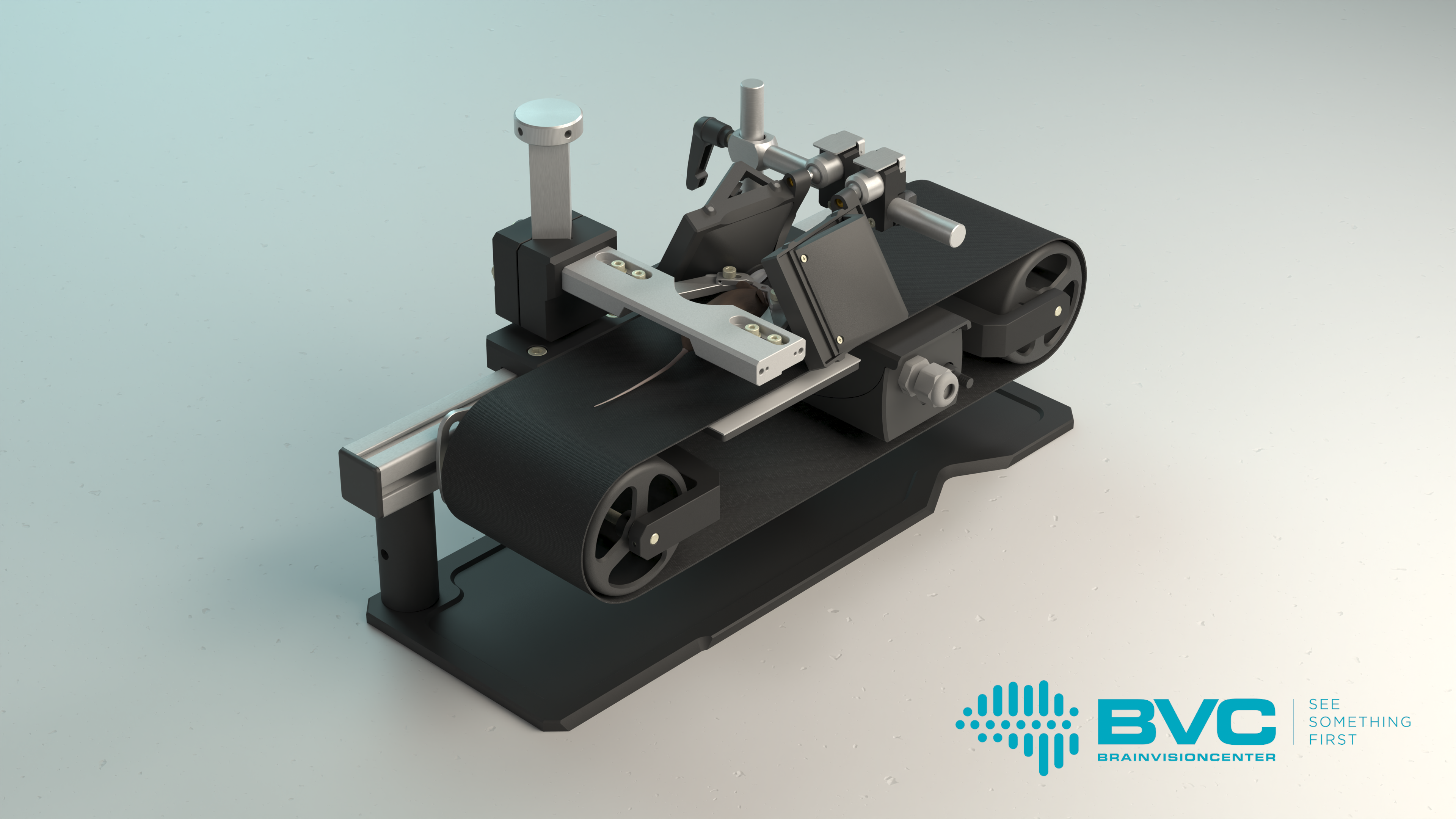
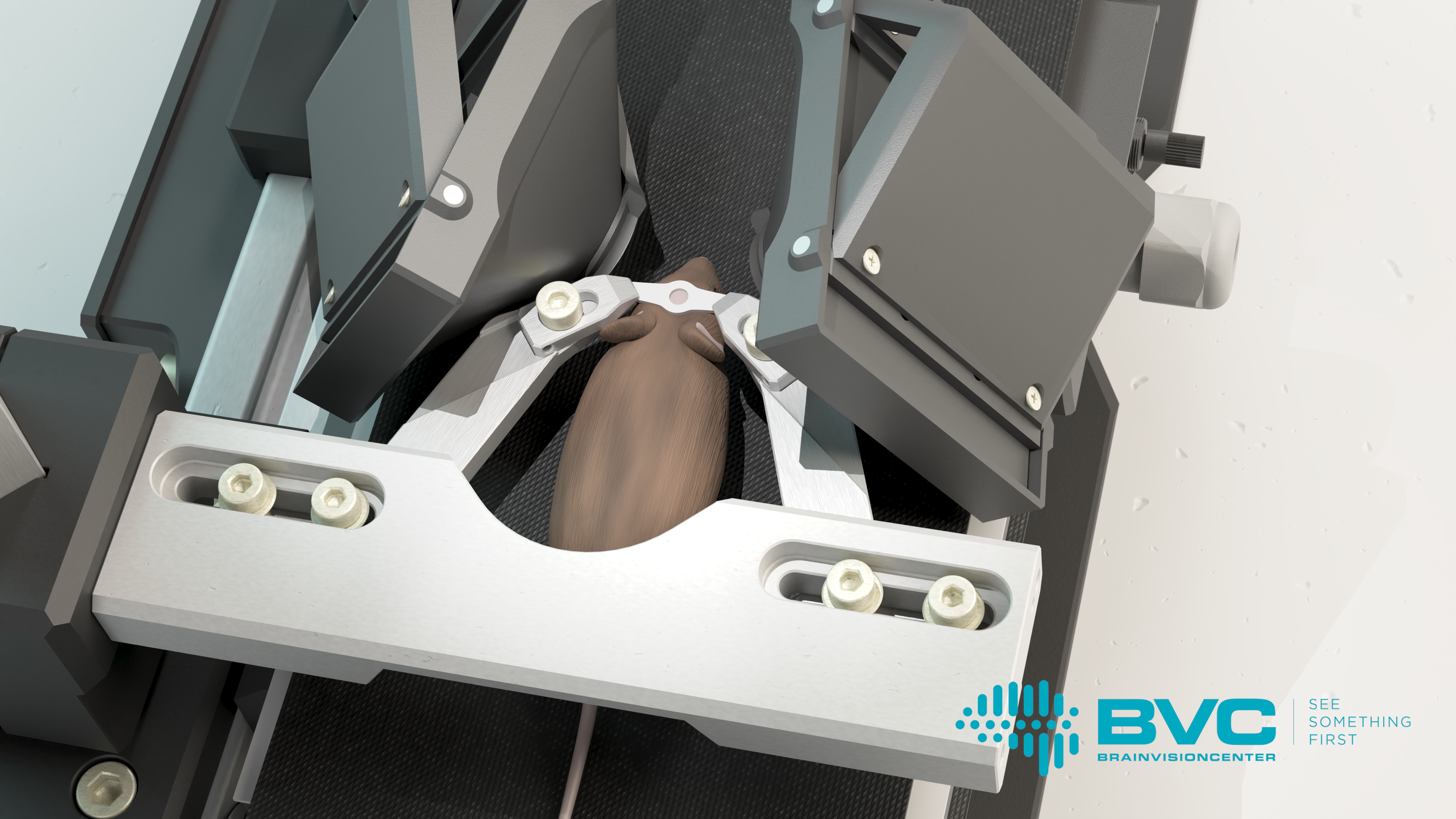
The Moculus VR system includes a specialized treadmill that records and transmits data on the mouse’s movements, two screens, and a matching optical imaging system. The latter provides the mice with a field of vision wider than 180 degrees, enabling them to interact naturally with the virtual environment. Meanwhile, researchers can map the mouse’s brain activity patterns using two-photon microscopy, which allows them to gain deeper insights into how animals learn and the neural mechanisms that govern decision-making. These studies not only advance our understanding of fundamental brain functions but also contribute to the development of therapeutic solutions for neurological disorders, such as vision impairment.
“Rodents’ visual learning abilities are surprisingly advanced. Contrary to previous assumptions, they can acquire new visual information in as little as one day, or sometimes just 30 minutes. This means rodents learn over 100 times faster with Moculus than with earlier virtual reality systems, which required 5–9 days of training. By reducing the difficulties and artifacts associated with lengthy training sessions, Moculus is revolutionizing the study of visual learning mechanisms, enabling discoveries even during a single short training session,” explained Dr. Linda Judák, lead researcher at HUN-REN KOKI and BVC. One of the device’s greatest advantage is its ability to identify complex brain activity patterns associated with learning, including so-called anticipatory neuronal signals that appear even before the presentation of visual stimuli. Dr. Linda Judák also added that during their research, they observed that neurons gradually become engaged in the learning process, with their activity intensifying particularly during the critical period preceding behavioral decisions. Using the device, they discovered new, previously unknown neural network mechanisms that emerge during visual learning.
According to current scientific literature, the activity of neurons in the visual cortex increases by approximately 10% as a result of learning. Moreover, the latest research has produced surprising findings: in some cases, a 0% change in activity or even a decrease in activity has been observed. It is important to note that in these earlier studies, training mice took 5–9 days, providing sufficient time for memory consolidation processes to reorganize brain activity patterns. Thanks to the ultra-fast learning opportunity provided by Moculus, researchers were able, for the first time, to capture a snapshot of brain activity before these reorganization processes began, directly observing the effects of learning. The results indicate that the functioning of the visual cortex differs significantly from the previously accepted textbook data. The brain is capable of activating nearly all neurons in the visual cortex for a short time to perform visual tasks, thereby maximizing its computational capacity to achieve a richer representation of visual components.
The essence of learning lies in the competition between these rich neuronal representations. During the learning process, a kind of “competitive process” occurs in the brain among spatiotemporal neuronal representations, aiming to encode behaviorally relevant information, such as positive or negative reinforcement. It has been demonstrated that the feedback derived from these signals is the critical information that teaches and reprograms the functioning of neural networks at the cellular level. This process determines the “winning” representation that dominates the encoding, while the coding of other information returns to baseline activity,” explained Dr. Balázs Rózsa, Director of BVC and Group Leader at HUN-REN KOKI and Pázmány Péter Catholic University.
He emphasized that the most significant achievement of the project is the new tool, which generates spatiotemporal brain activity patterns that encode specific visual elements of our environment with orders of magnitude greater precision and depth. This enables vision restoration devices based on 3D acousto-optical microscopy to reactivate neuronal activity with unprecedented accuracy, thereby creating much more precise artificial vision than ever before.
“Three years ago, at the end of 2021, during the establishment of the BrainVisionCenter, Botond Roska and I identified as our main mission, the basic research in vision restoration, the development of specialized research tools necessary for this, and cortical vision restoration related research. Moculus represents an important milestone in these efforts, as it allows the cortical role of the genetic engineering methods developed by Botond and his team to be tested more effectively than with previous methods,” emphasized the director.
The device has generated significant interest in the field of neuroscience research tools, as no similar solution is currently available on the market. One of its major advantages is its compact, modular design, which makes it easily adaptable to any electrophysiological or imaging equipment, such as two-photon microscopey system.